Glutamine synthetase (GS) catalyzes the reaction of glutamate and ammonia to form glutamine through a phosphorylated intermediate. Creatine kinase (CK) catalyzes the transfer of a phosphate group from ATP to creatine to yield phosphocreatine. Although GS and CK catalyze similar reactions, they have no significant sequence similarity.
Liaw and Eisenberg solved crystal structures of GS to elucidate the mechanism of glutamine synthesis and to identify residues involved in ATP binding and in transfer of the gamma phosphate. One such structure was superimposed with a CK structure to generate a family of several hundred structure superpositions. The above tools were used to examine the ATP-binding residues of GS and CK. While no crystal structures of CK bound with MgATP or substrate are available, the studies suggest that many of the ATP-binding residues in GS have potential homologs in CK.
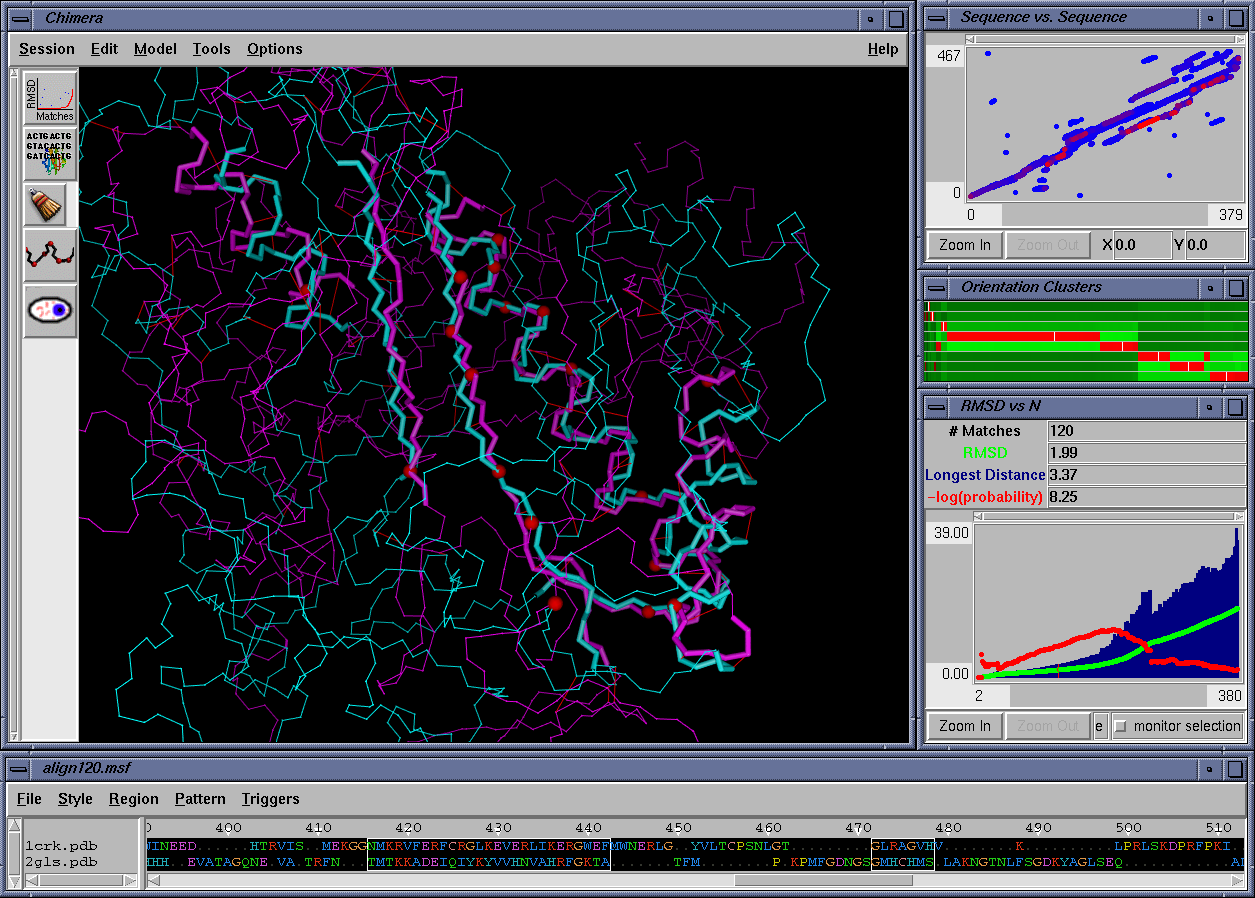
In the figure above, the superposition that matches 120 residues from each structure is displayed in the graphics window. The "RMS vs. N" plot both summarizes data from the entire family of superpositions, and displays quantitative measures of the currently selected superposition (e.g., RMSD and -log(probability) as defined by Gerstein and Levitt). The "Orientation Clusters" plot displays a clustering of all superpositions in the family. The "Sequence vs. Sequence" plot displays which residues from each structure are likely to be matched to one another. Finally, the "align120.msf" window displays the current superposition in a sequence alignment format.
©2004 The Regents, University of California; all rights reserved.